Beer's Lambert Law Equation
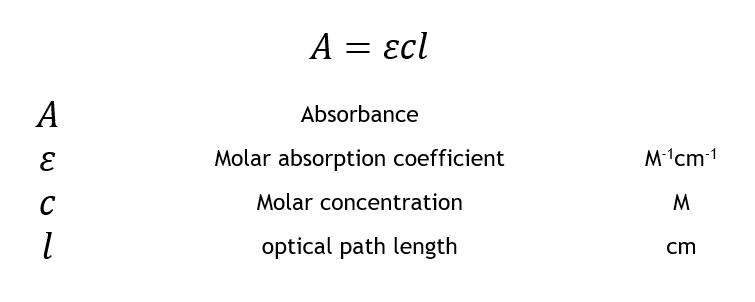
MathrmA varepsilon bc nonumber. Absorbance is defined as the logarithm of the ratio of incident to transmitted radiant power through a sample excluding the effects on cell walls.

Beer Lambert Law Labster Theory
The Beer-Lambert law relates the attenuation of light to the properties of the material through which the light is traveling.
. Molarity M - moles of soluteliters of solution not solvent. Alternatively for samples which scatter light absorbance may be defined as the negative logarithm of one minus absorptance as measured on a uniform sample. Beers law is applicable only in cases where.
The Beer Lambert Law When a monochromatic light of initial intensity Io passes through a solution in a transparent vessel some of the light is absorbed so that the intensity of the transmitted light I is less than Io There is some loss of light intensity from scattering by particles in the solution. The length of the light path is known. Limitations of Beer Lamberts Law.
There is a fee for seeing pages and other features. We hope now that you are confident about Beers law and its significance. Basically Pierre Bouger discovered the law in 1729 and published it in Essai DOptique Sur La Gradation De La Lumière.
The two laws may be combined to write. A ε b c Where ε is the wavelength-dependent molar absorptivity coefficient with units of M-1 cm-1. Beers Law is also known as the Beer-Lambert Law the Lambert-Beer Law and the BeerLambertBouguer LawThe reason there are so many names is because more than one law is involved.
Beers Law and Lamberts law. Papers from more than 30. Absorbed as described by the Beer-Lambert equation also known as Beers Law.
According to Beer-Lambert Law. Mass Percent - mass solutemass solution x 100 mass units are the same unit for both solute and solution. Check out the derivation of Beer-Lambert law.
When working in concentration units of molarity the Beer-Lambert law is written as. Since the concentration path length and molar absorptivity are all directly proportional to the absorbance we can write the following equation which is known as the Beer-Lambert law often referred to as Beers Law to show this relationship. Formulated by German mathematician and chemist August Beer in 1852 it states that the absorptive capacity of a dissolved substance is directly proportional to its concentration in a solution.
Article Beers Law Why Absorbance Depends Almost Linearly on Concentration. It refers to a device which helps specific solutions to absorb a particular wavelength of light. The representation of Beer Lamberts law is given as A ABC.
As in the case of Lamberts law equation 9 may be transformed into log I Io 𝑐 𝑥 11. This is known as the Beers law. This expression relates the absorbance to the solute concentration in a given solution A eLc 1 where A is the compounds absorbance unitless e is the molar absortivity Lmolcm L is the path length cm and c is the compound concentration molL.
The term is used in many technical areas to quantify the. The concentration of the solution does not cross 001M. Beers Law relates to two variables input intensity I 0 and output intensity I.
Beers law states that the intensity of light decreases concerning _____ a Concentration b Distance c Composition d Volume. Beer-Lambert Law demonstrates the linear relationship between the absorbance of light and the concentration of a substance. The solution can absorb the light that is incident onto it.
It is the absorbance of a substance placed in 1cm cuvette cell when the concentration is 1 molar. Beer-lambert law describes the link between the attenuation of light through. Beer-Lambert Law is a combination of two laws.
Newtons law of cooling defines the rate at which an exposed body changes temperature by radiation which is roughly equal to the difference in temperature between the item and its surroundings provided. Mass Concentration kgm 3 or gL - mass of solutevolume of solution. This is just saying that the output intensity will be the same or lower compared with the input intensityThis coefficient in the exponential function µ pronounced mu is dependent on the material and the energy of the x-rays.
The Beer-Lambert law known by various names such as the Lambert-Beer law Beer-LambertBouguer law or the Beers law. The colorimeter is usually used to measure the concentration of a known solute in a given solution with the help of the Beer-Lambert law. Beer lambert Law 1.
Beers law also called Lambert-Beer law or Beer-Lambert law in spectroscopy a relation concerning the absorption of radiant energy by an absorbing medium. Of the Beer-Lambert law -Log 10 II 0 epscd both sides of the equation are generally incorrect. The colorimeter was invented in the year 1870 by Louis J Duboscq.
The multiplying term here will always be a number between 0 and 1. Other Names for Beers Law. Newtons Law of Cooling.
This page takes a brief look at the Beer-Lambert Law and explains the use of the terms absorbance and molar absorptivity relating to UV-visible absorption spectrometry. Beer-Lambert law explains the relationship between absorbance path length and concentration of a given material. TThhee BBeeeerr LLaammbbeerrtt LLaaww 2.
It is used in analytical chemistry to measure the absorbance of several samples. Lamberts law states that the intensity of light decreases concerning _____ a Concentration b Distance c Composition d Volume. Normality N - grams active soluteliters of solution Molality m - moles of solutemass of solvent not mass of solution.
When the concentration c is expressed in mol L b is called the molar absorption co-efficient. Newton was the first to analyze the relationship between the heat lost by a body in a certain enclosure and its temperature systematically.
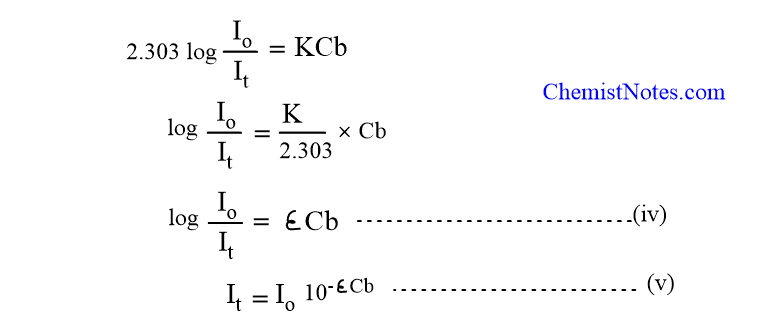
Beer Lambert Law Derivation Deviation Application And Limitations Chemistry Notes

Beer Lambert Law Transmittance Absorbance Edinburgh Instruments


No comments for "Beer's Lambert Law Equation"
Post a Comment